
Blog
Articles of Persia Paya Mining Articles of Persia Paya Mining Articles of Persia Paya

Magnesium sulfate (or magnesium sulphate) is an inorganic salt (chemical compound) containing magnesium, sulfur and oxygen, with the formula MgSO4. It is often encountered as the heptahydrate sulfate mineral epsomite (MgSO4·7H2O), commonly called Epsom salt, taking its name from a bitter saline spring in Epsom in Surrey, England, where the salt was produced from the springs that arise where the porous chalk of the North Downs meets non-porous London clay. The monohydrate, MgSO4·H2O is found as the mineral kieserite. The overall global annual usage in the mid-1970s of the monohydrate was 2.3 million tons, of which the majority was used in agriculture Uses Medical: Magnesium sulfate is a common mineral pharmaceutical preparation of magnesium, commonly known as Epsom salt, used both externally and internally. Epsom salt is used as bath salts and for isolation tanks. Oral magnesium sulfate is commonly used as a saline laxative or osmotic purgative. Magnesium sulfate is the main preparation of intravenous magnesium. Agriculture: In gardening and other agriculture, magnesium sulfate is used to correct a magnesium or sulfur deficiency in soil; magnesium is an essential element in the chlorophyll molecule, and sulfur is another important micronutrient.[22] It is most commonly applied to potted plants, or to magnesium-hungry crops, such as potatoes, roses, tomatoes, lemon trees, carrots, and peppers. The advantage of magnesium sulfate over other magnesium soil amendments (such as dolomitic lime) is its high solubility, which also allows the option of foliar feeding. Solutions of magnesium sulfate are also nearly neutral, as compared to alkaline salts of magnesium, as found in limestone; therefore, the use of magnesium sulfate as a magnesium source for soil does not significantly change the soil pH.
Read More
Heavy elements as toxic elements for animals and plants in magnesium carbonate and oxide have to be very low in content and our products overcome the standard factors!
Read More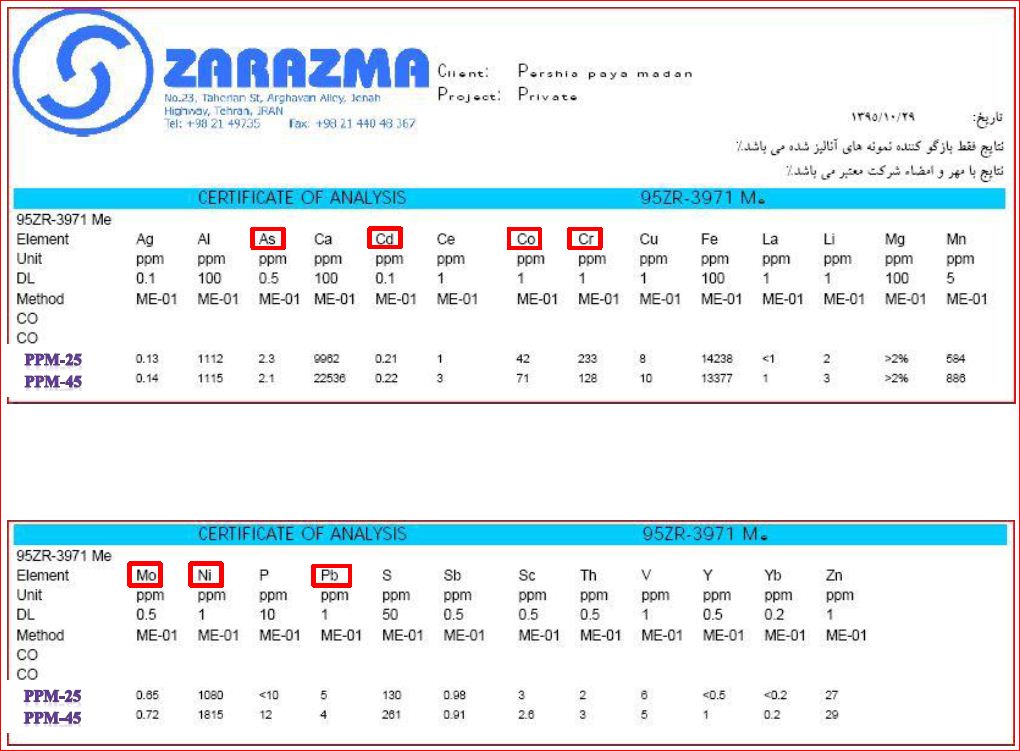
According to specific conditions for livestock and poultry feed market, different magnetite products are classified for different applications which are presented on attached table. Due to low levels of heavy metals in our products (see Table attached), we are proud of supplying a wide range of Livestock and Poultry feed market and with supplementation of a high standard, homogeneity and stable conditions.
Read More
Magnesite is a mineral with the chemical formula MgCO3 (magnesium carbonate). Mixed crystals of iron(II) carbonate and magnesite (mixed crystals known as ankerite) possess a layered structure: monolayers of carbonate groups alternate with magnesium monolayers as well as iron(II) carbonate monolayersManganese, cobalt and nickel may also occur in small amounts. Occurrence Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites often are cryptocrystalline and contain silica in the form of opal or chert. Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide within groundwaters. Formation Magnesite can be formed via talc carbonate metasomatism of peridotite and other ultramafic rocks. Magnesite is formed via carbonation of olivine in the presence of water and carbon dioxide at elevated temperatures and high pressures typical of the greenschist facies. Magnesite can also be formed via the carbonation of magnesium serpentine (lizardite) via the following reaction: serpentine + carbon dioxide → talc + magnesite + water 2 Mg3Si2O5(OH)4 + 3 CO2 → Mg3Si4O10(OH)2 + 3 MgCO3 + H2O. However, when performing this reaction in the laboratory, the trihydrated form of magnesium carbonate (nesquehonite) will form at room temperature. This very observation led to the postulation of a "dehydration barrier" being involved in the low-temperature formation of anhydrous magnesium carbonate. Laboratory experiments with formamide, a liquid resembling water, have shown how no such dehydration barrier can be involved. The fundamental difficulty to nucleate anhydrous magnesium carbonate remains when using this non-aqueous solution. Not cation dehydration, but rather the spatial configuration of carbonate anions creates the barrier in the low-temperature nucleation of magnesite Magnesite has been found in modern sediments, caves and soils. Its low-temperature (around 40 °C) formation is known to require alternations between precipitation and dissolution intervals. Magnesium-rich olivine (forsterite) favors production of magnesite from peridotite. Iron-rich olivine (fayalite) favors production of magnetite-magnesite-silica compositions. Magnesite can also be formed by way of metasomatism in skarn deposits, in dolomitic limestones, associated with wollastonite, periclase, and talc.
Read More
Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites often are cryptocrystalline and contain silica in the form of opal or chert. Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide within groundwaters. Similar to the production of lime, magnesite can be burned in the presence of charcoal to produce MgO, which, in the form of a mineral, is known as periclase. Large quantities of magnesite are burnt to make magnesium oxide: an important refractory material used as a lining in blast furnaces, kilns and incinerators. Calcination temperatures determine the reactivity of resulting oxide products and the classifications of light burnt and dead burnt refer to the surface area and resulting reactivity of the product, typically as determined by an industry metric of the iodine number. Magnesite has been used in flooring as a binder. It is used as a lining in furnaces and cement kilns. It also makes an excellent firebrick. Caustic calcined magnesia is used as a food supplement in fertilizers and for fillers in plastics, paints and paper. It is also used as a filler and catalyst in the production of synthetic rubber. Raw magnesite is used for surface coatings and as a fire retardant. It is also used in ceramics, face powder, disinfectants, Epsom salts and boiler wrappings. Main applications: Agricultural Applications Electric Grade Steel Electric Utilities Flame Retardant / Smoke Suppressant Food Additives Glass Making H2S Odor and Corrosion Control Refractories (Hard Burned Magnesium Oxide) Industrial
Read More
Heavy element content Considering the specific requirements of defining the products for the livestock and poultry market after consulting the technical and commerce section of the annex for this market was designed Given the low amounts of heavy metals in products Appendix Table we are proud to provide the market for animal feed and complementary products with a high standard of production uniformity Our two best-selling products in Animal Feeding market include magnesium carbonate PPM-25 and magnesium oxide CCM PPM-45 whose analysis of heavy elements is as follows
Read More
Magnetite is relatively frequent as a mineral, there are, however, only two types of economically important deposits: The worlds largest reserves are found in deposits of the Veirsch type. These are strata-bound lensoid and nearly monomineralic bodies consisting of coarsely crystalline ,,spar,, magnesite hosted by marine platform sediments. Conspicuous are sedimentary and diagenetic features of the magnesite bearing suites, which indicate formation within a subtidal, evaporitic environment closely associated with sabkhas, mudbars or other inter- to supratidal highs. The coarse crystallinity of these magnesites is a product of diagenesis. As spar magnesites as a rock are mainly characterized by diagenetic features it is now often concluded that the main phase of magnesium concentration would also have taken place during late diagenesis or early metamorphism from fluids formed by these processes. In that case, their origin would be by hydrothermal replacement. Contrary to this trend it is here concluded by integrating important data that Veitsch type magnesites are essentially of syngenetic origin. A genetic model is presented, which stresses their kinship with shallow water marine chloride type evaporites. Deposits of cryptocrystalline magnesite of the Kraubath type are much smaller and less frequent than spar magnesites, although they are quite important because of their high quality product. Deposits include veins and stockworks of snow-white magnesite ii) ultramafic country rocks. Genetic models of Kraubath type magnesites include the extremes of supergene alteration versus hypogene-hydrothermal formation.
Read More
Magnesium oxide, or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium. Formula: MgO Molar mass: 40.3044 g/mol IUPAC ID: Magnesium oxide Melting point: 2,852 °C Density: 3.58 g/cm³ Boiling point: 3,600 °C Magnesia or magnesium oxide is an alkaline earth metal oxide. The majority of magnesium oxide produced today is obtained from the calcination of naturally occurring minerals. Magnesite, MgCO3, being the most common. Both MgCO3 and Mg(OH)2 are converted to MgO by calcination. The thermal treatment of the calcination process affects the surface area and pore size and hence the reactivity of magnesium oxide formed. The source largely determines the level and nature of impurities present in the calcined material. Caustic calcined magnesia is formed by calcining in the range 700 – 1000°C. By calcining in the range 1000 – 1500°C the magnesium oxide is used where lower chemical activity is required e.g. fertiliser and animal feed. Dead-burned magnesia, which is produced in shaft and rotary kilns at temperatures over 1500°C, has reduced chemical reactivity therefore is more suited to refractory applications. Fused magnesia is used for a variety of refractory and electrical applications. This type of magnesia is produced in an electric arc furnace from caustic calcined magnesia at temperatures in excess of 2650°C Key Properties There are few dense engineering ceramics of the structural type made from pure magnesia. However there is a wide range of refractory and electrical applications where magnesia is firmly established. The properties of major interest are as follows: Good Refractoriness Good Corrosion Resistance e.g. Basic Steelmaking Slags, Sodium Hydroxide, Fe, Co, Ni High Thermal Conductivity Low Electrical Conductivity Transparency to Infrared Applications Magnesium Oxide in Animal Food Supplements Magnesium is an essential nutrient for all animals, serving a variety of vital functions within the body PPM magnesium oxide, a granular, high purity feed grade magnesium oxide processed from natural magnesite, is an excellent source of magnesium for cattle consuming low-magnesium forages. Inadequate magnesium in the diets of grazing animals can cause a serious metabolic disorder known as “Grass Tetany.” The rapid growth rate of grass in Spring means that pastures are often lacking in magnesium. Young grass is higher in simple carbohydrates and protein, but lower in most minerals. Grass tetany occurs when the level of magnesium in blood falls below a critical threshold. Providing a palatable magnesium supplement with high biological availability is an effective strategy for maintaining magnesium requirements of grazing animals. In dairy cattle, magnesium also helps raise the butter fat content of the milk and neutralizes acidosis caused by high amounts of grain in their diet. The balance of magnesium performs an important role in utilization of calcium, which is released from bone into the bloodstream. As with calcium, magnesium is classified as a macro mineral, which means that it is required by the body in reasonably large quantities. Magnesium is also important in converting sugars into energy. Refractories Magnesia is widely used in the steel industry as a refractory brick. It is often impregnated with carbon (tar, pitch, graphite) to provide the best properties for corrosion resistance in environments of basic slags, particularly in BOF furnaces or slag lines of treatment ladles. Magnesia bricks often in combination with spinel or chrome are also used in ferroalloy, non-ferrous, glass and cement industries. Castables and sprayables based on magnesia are widely used for basic refractory linings for steel transfer applications. The lime to silica ratio present in the magnesia has a major influence on its properties. Cements Magnesia (or Sorel) cement is a refractory binder based on a magnesium oxychloride formulation. It is fast-hardening and has a number of refractory and general repair applications. Magnesia is also used as a room temperature curing agent for phosphate cements. Heating Elements Magnesia powder is widely used as a filling for electrical heating elements for applications in contact with air or liquids. Fused magnesia has the ideal combination of electrical resistance and thermal conductivity. The MgO forms a layer between the element and the outer sheath. It is also used as mineral insulation in cables. Brake Linings Magnesia has been included in brake linings due to its thermomechanical properties. Its intermediate hardness gives sufficiently low wear on metal while conducting heat from the friction contact surfaces.
Read More
Agricultural http://blog.magspecialties.com/category/applications/agricultural/ Electric Grade Steel http://blog.magspecialties.com/category/applications/electric-grade-steel/ Electric Utilities http://blog.magspecialties.com/category/applications/electric-utilities/ Flame Retardant / Smoke Suppressant http://blog.magspecialties.com/category/applications/flame-retardant-smoke-suppressant/ Food Additives http://blog.magspecialties.com/category/applications/food-additives/ Glass Making http://blog.magspecialties.com/category/applications/glass-making/ H2S Odor and Corrosion Control . . . coming soon Industrial Applications . . . coming soon Mining http://blog.magspecialties.com/category/applications/mining/ Pulp Bleaching http://blog.magspecialties.com/category/applications/pulp-bleaching/ Refractories and Steelmaking http://blog.magspecialties.com/category/applications/refractoroes-and-steelmaking/ Rubber and Plastics http://blog.magspecialties.com/category/applications/rubber-and-plastics/ Wastewater and Water Treatment http://blog.magspecialties.com/category/applications/wastewater-and-water-treatment/
Read More
Magnesium oxide and magnesium hydroxide can both be used in various ways for the treatment of wastewater In order to repel the unpleasant odor from waste water its main sources H2S and ammonia must be eliminated. This can be achieved by the introduction of MgO in the system with a buffer solution of 9-9,5 pH prohibiting in this way the formation of H2S. Corrosion of the utilities networks or Struvite tanks is also attributed to the formation of sulfuric acid (H2SO4) alkaline magnesium reagents can help in their protection either with continuous addition magnesium oxide/hydroxide in parts of the network for pH adjustment or through coating the free surface of the pipes with magnesium hydroxide slurry. The removal of phosphorus is of major interest lately since phosphates interfere with the phenomenon of eutrophic and lead to the death of aquatic life. Additionally, due to the decrease of natural phosphate deposits the recovery and recycling of phosphorous has become desirable. The byproduct of the phosphorus removal is called struvite and could be used as a fertilizer (Mg, N, P source). Magnesium ions provoke the increase of the chlorophyll production since they occupy the central position in its molecule, while N and P are important nutrients for the plants. In that way the treatments byproduct can be sold as fertilizer making the whole treatment process even more profitable. It should be noted that the use of MgO / Mg(OH)2 achieves the best possible recovery yield of the phosphates in comparison to Ca(OH)2/CaO due to more effective pH control.
Read More
Magnesium is an essential nutrient for all animals, serving a variety of vital functions within the body. Inadequate magnesium in the diets of grazing animals can cause a serious metabolic disorder known as “Grass Tetany.” The rapid growth rate of grass in Spring means that pastures are often lacking in magnesium. Young grass is higher in simple carbohydrates and protein, but lower in most minerals. Grass tetany occurs when the level of magnesium in blood falls below a critical threshold. Providing a palatable magnesium supplement with high biological availability is an effective strategy for maintaining magnesium requirements of grazing animals. In dairy cattle, magnesium also helps raise the butter fat content of the milk and neutralizes acidosis caused by high amounts of grain in their diet. The balance of magnesium performs an important role in utilization of calcium, which is released from bone into the bloodstream. As with calcium, magnesium is classified as a macro mineral, which means that it is required by the body in reasonably large quantities. Magnesium is also important in converting sugars into energy.
Read More
